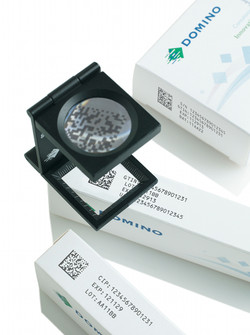
Viewpoint: A shot in the arm for generics producers
Domino inksThere is nowhere to hide from the Falsified Medicines Directive. It impacts generics producers in the same way as any other drug manufacturer or re-packager selling into Europe. With just under 48 months to go before the deadline, which is likely to be mid-2018, the clock is ticking for all producers to get their house in order.
The subject was hot on the agenda at the European Generics Association (EGA) meeting in Madrid earlier this month. As generics manufacturers often supply products with much lower margins than patented drug producers, their budgets are restricted when it comes to new investment – which is why many of them have been holding out until the last minute to find the ‘best-fit’ solution for their businesses.
Related Posts
Two men arrested by HMRC as part of investigation into suspected multi-million pound tobacco smuggling fraud
A MAN from Dagenham was arrested by HMRC last week as part of an investigation...
China’s fake milk powder scandal
Chaotic sales channels have been blamed for China’s latest fake infant formula...
Telangana: DCA busts illegal drug manufacturing; medicines licenced as food products; stocks Seized
The Drugs Control Administration (DCA), Telangana, in a recent series of...
Assam: Massive consignment of foreign-origin cigarettes seized in Cachar
Guwahati: A massive consignment of foreign cigarettes was seized by the police...






