Multi-factor authentication market to reach $5.45 billion by 2017
The two-factor authentication market will touch $5.45 billion by the year 2017, predicts MarketsandMarkets. The report looks at the market by authentication type, application and geography and predicts a compound annual growth of 17.3%. Multi-factor authentication refers to user and employee authentication that uses two or more verifying authentication techniques – hardware token, one-time password (OTP), password/PIN, biometrics, etc. Multi-factor authentication has become an integral part of personal and enterprise security due to increase in unauthorized access, fraud, border intrusion and more. To account for this, multi-factor authentication is being deployed at all the major security checkpoints at airports, commercial complexes, retail malls, banks, financial institutions and others. Multi-factor authentications value lies in its ability to provide accurate authentication along with a minimal margin of duplicity or error.
Lear MorePolice crack diploma counterfeiting groups
Chinese police have cracked two major diploma counterfeiting groups and detained at least 21 suspects, said railway police on Friday. Shanghai railway police raided two locations involved in counterfeiting diplomas and detained 21 suspects in late July, said a statement from the railway police division of the Ministry of Public Security. Police confiscated more than 40,000 fake seals of government departments and other institutions, and more than 50,000 fake diplomas at the locations, the statement said. Last week, Chengdu railway police raided another counterfeiting location and seized about 2,700 fake diplomas and 3,190 fake seals following a lead from a train passenger using a fake identification card, it said.
http://www.china.org.cn/china/2013-08/23/content_29807500.htm
Lear MoreAnti-counterfeiting: customs brand registration
The global trade in counterfeit goods has reached staggering proportions, growing 10 000% over the past two decades, and resulting in a highly-profitable, illegal industry worth between R1.6 trillion and R4.8 trillion annually. To put this in perspective, this amounts to roughly 8% of world trade. Unfortunately, South Africa has become a major target for counterfeiters, with counterfeit goods in excess of R600 million being seized since 1997.Although there is a widespread impression that counterfeiting is a victimless crime, this is far from the truth. Apart from tax revenue lost, trade in counterfeit goods results in massive job losses, and erodes the market for genuine products. Closer to home, the consumption of counterfeit goods poses a real danger to human health and safety, as counterfeiting expands beyond the traditional bounds of luxury goods, DVD’s, clothing and cigarettes, to children’s toys, automotive parts, food products, and pharmaceuticals. From a brand protection perspective, counterfeiting impacts detrimentally on reputation and goodwill, in addition to causing a loss in sales and profits. Almost any conceivable product is fair game to counterfeiters, and the more popular and successful a brand becomes, the more appealing it becomes to counterfeiters.
Lear MoreHotels, bars told to remove liquor bottle labels after use
PUNE: The excise department of Pune has directed over 100 restaurants and bars in the district to scrape off labels from used bottles of Indian Made Foreign Liquor (IMFL) or mark a cross on the label on each side of the used bottle to prevent them from being reused to sell spurious liquor.
The directive, which came after instances of bootlegging were reported in the state, will also involve excise inspectors making mandatory checks in five-star hotels twice a month to make sure all used imported foreign liquor bottles are smashed and not sold to scrap dealers. The excise inspectors were also instructed to bring the used bottles in front of them during the routine inspections at permit rooms, bars, clubs and five-star hotels. Five star hotels, where imported liquor is sold extensively, will in fact, have excise officials checking twice every month to make sure that imported liquor bottles are being smashed after use.
Lear MoreOver 6,000 arrested in Interpol fake-goods sweep
PARIS — More than 6,000 people around the world were arrested in a two-month anti-counterfeiting sweep that netted tens of millions of dollars worth of fake shampoo in China, phony cigarettes in Turkey and bogus booze in Chile, Interpol said Thursday. In all, the operations coordinated by the Lyon, France-based international police agency in May and June seized some 24 million fake goods worth nearly $133 million, Interpol said in a statement. The combined haul ranks among the largest operations ever conducted by the agency’s special anti-counterfeiting unit, according to its director, Michael Ellis. As part of the worldwide push against counterfeiting, the agency helped lead operations by local authorities in the Americas, Africa, Europe and, for the first time, in Asia. The Asian operation shut down 21 production sites operated by eight criminal syndicates making fake shampoo and toothpaste in southern China. More than 400 people were arrested in Thailand for hawking counterfeit clothing and DVDs, while in Vietnam police arrested an individual linked to $6 million worth of illicit electronic appliances. Ellis highlighted the significance of the Chinese police cooperation for the first time. “We reach out to various national police forces on a regional basis. This time the police in China joined the operation, with great effect,” he said.
http://www.azcentral.com/news/free/20130718interpol-counterfeit-goods-sweep-arrests-france.html
Lear MoreFeds: Counterfeit submarine parts shipped to Groton base
Groton — The U.S. Naval Submarine Base on at least three separate occasions received counterfeit semiconductors from Hong Kong or China, though it is unclear whether any of the components wound up on a nuclear submarine.According to the indictment this week of a military-components distributor who was charged on counts of conspiracy, fraud and trafficking, Peter Picone, 40, of Methuen, Mass., and unidentified co-conspirators shipped the counterfeit integrated circuits to the sub base between November 2011 and February 2012. At least two of the circuits were intended for active-duty nuclear submarines in Groton, though there was no indication in the indictment whether they actually had been used on board a sub. One was intended for an alarm panel, while another was to be used in a radio-transmission test, the indictment said. “I have to buy China and risk fake parts to compete. … It’s my whole biz,” the indictment quotes Picone as saying in a 2008 instant message. The indictment also tied Picone — who owns Epic International Electronics and once headed electronics distributor Tytronix Inc., both based in Massachusetts — to the shipment of other counterfeit products from Hong Kong or China to unidentified Connecticut customers. In addition, a defence contractor in Florida bought 33 integrated circuits from one of Picone’s companies to be used during repair work on an active-duty nuclear submarine’s secondary propulsion system, the indictment said.
http://theday.com/article/20130716/NWS09/130719772/1017
Lear MoreNHRC seeks report from health director on death due to ‘fake’ brain shunt
The National Human Rights Commission had sought an action-taken report from the director, health and family welfare, Chandigarh, into the death of Gurjot Kaur, a four-month-old girl who was fitted a “counterfeit” shunt during a brain surgery at the Post-Graduate Institute of Medical Education and Research (PGIMER). The director is to comply within six weeks. Gurjot’s SAS Nagar-based family had approached the national panel headquartered at New Delhi via a complaint by city-based advocate and social activist Pankaj Chandgothia. It detailed how Gurjot had been operated upon on April 2, got a “counterfeit” shunt, and died on June 21 at the PGIMER. “With this, the parents lost their only child to medical malpractices at PGIMER and its chemists,” alleged Chandgothia. Going further, Chandgothia has alleged large-scale malpractices at the institute and chemists. In the complaint, “Thus, we have sought action against the PGIMER Director, Baby Care Chemists on the campus and the director of health services, Chandigarh.” The UT’s health department director and the PGIMER director “are also liable for criminal negligence… They have failed to monitor and regulate the sale of fake medical equipments and expired medicines”.
Lear More
Duplicate cement seized
Police seized 1.5 tonnes of duplicate cement packed in 300 bags and arrested the driver of the truck carrying it at Chagharia Chowk on NH-5(A) under Kendrapara Sadar police limits on Wednesday night. Sources said a racket selling duplicate cement is operational in Kendrapara and its nearby areas. The gang uses bags of reputed companies to fool customers and sell the cement at a lower rate, said Tapas Pradhan, IIC of Kendrapara Sadar police station. Following a tip-off from a trader a Marsaghai -bound truck that was unloading such cement at various places was stopped and taken to police station. The driver could not produce papers to show that the cement bags were of a particular company, added the police officer. The driver used to bring such cement from Jagatapur to be sold in Kendrapara and its nearby areas.
Lear More‘Fake fragrances [in Dubai] damage brands’
The scent of counterfeit fragrances could create a stink for big brands in the UAE, according to a retail branding expert. Some Dubai stores are playing on the popularity of tech giants Samsung, BlackBerry and facebook, and luxury auto maker Bentley, by selling fragances carrying logos of the leading firms. “This is definitely not a good thing for the brands because they don’t know the contents of the bottles, and whatever is in them is out of their control,” said Ashish Panjabi, COO of Jacky’s. Panjabi, whose multi-brand retail chain has received ‘Superbrand’ status for eight consecutive years since 2004 from the UAE Superbrands Council, added: “Most perfumes contain chemicals, but we don’t know if these fakes contain any toxic or expired substances. If users end up having any health implications, that’s really not something a brand wants their name to be associated with.” Panjabi was not surprised, however, that imitators are cashing in on the popular names, pointing out that luxury brands such as Bentley – one of names being used by counterfeiters – would hardly want their posh motor linked to a low-cost spray. Asked what he would do if he saw a ‘Jacky’s perfume’ come on the market, he said: “We would take immediate action as we have a registered trademark.”
Lear More
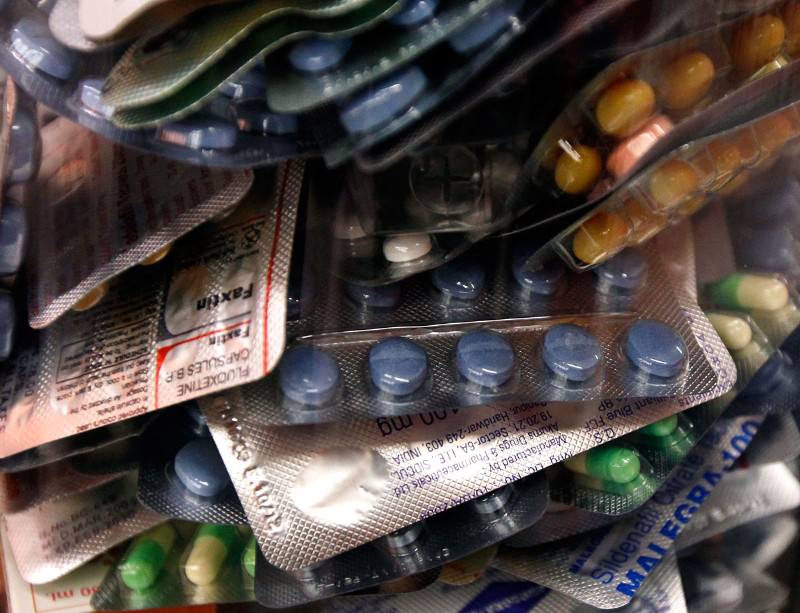
Global crackdown seizes fake drugs worth $41 million
A worldwide police crackdown has seized a record $41-million haul of illegal pharmaceuticals being sold online. Nearly half of the 9.9 million doses of illegal drugs were seized in the UK. The UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) said the haul involved drugs for slimming, hair loss and erectile dysfunction. Worldwide, the haul included more potentially life-threatening counterfeits, such as antibiotics and antivirals, and drugs for breast cancer, asthma, diabetes and epilepsy. The crackdown, called Pangea VI, was the sixth and biggest in an annual series organised by Interpol. It saw 99 countries netting nearly three times as many counterfeit pills as last year’s crackdown, with four times the value. Some 10,000 online pharmacies were shut down, and 58 people arrested, after Interpol tracked them via internet service providers, online payment systems, postal inspections and police raids. Most of the UK haul was made up of “unlicensed” drugs. These can be authentic pills, repackaged to conceal the fact that they are outdated, stolen, made by a manufacturer other than the one on the label or were intended for sale in other countries. Only 2.6 per cent of the 3.7 million pills seized in the UK in the latest sweep were outright fakes, says the MHRA – mock-ups of commercial brands minus the active ingredients.
http://www.newscientist.com/article/dn23769-global-crackdown-seizes-fake-drugs-worth-41-million.html
Lear More


