
Counterfeit CDs worth £245,000 seized at Manchester Airport
The country’s biggest ever haul of counterfeit CDs has been seized at Manchester Airport in a multi-force crackdown on a major international smuggling scam.The 1.2-tonne consignment of music, with a street value of £245,000, was smuggled in from Hong Kong.It included Rolling Stones, Queen and Taylor Swift albums – destined to be passed off as legitimate and sold on the internet over Christmas.Officers say the discovery has smashed a large-scale international counterfeit scam, believed to date back a decade, and worth more than £20m.Manchester Airport Border Force officers found the CDs – which were passed off as MP3s on paperwork – when they inspected freight that had arrived.Their find was the culmination of a joint investigation by the airport’s Border Force officers, the City of London Police FraudInvestigation team and anti-piracy investigators BPI and IFPI.A further 20,000 CDs were also seized by City of London Police officers from a warehouse in Lancashire and a man from Morecambe interviewed as part of an ongoing investigation.DI Kevin Kirton, who headed up the fraud investigation team, said their suspicions were alerted when the smuggler started selling CDs on well-known internet sites, under-cutting major firms by about 50p.He said: “His competitors were wondering how he could sell at those prices. This was a major international business exporting across the world.”After gathering evidence for six months, a delivery was tracked down to Manchester Airport.DI Kirton added: “We were surprised by the extent of the counterfeit CDs and I would call this just a snapshot of what’s been going on. We think it’s been going on for a decade, probably selling CDs worth more than £20m.”He said the smuggler had cleverly mixed in genuine CDs with the counterfeit to cover his tracks – and some of customers would have received the real thing.“They were being sold in such a way so customers would think it was a legitimate bargain.”“It feels good to have been involved in an operation that protects the public who may have been duped, especially at this time of year when budgets are so tight.”Border Force and Trading Standards are warning festive shoppers to be careful about what they buy and where they buy it from to avoidfuelling the illegal trade.In a separate operation, officers found Manchester was also the intended target city for £5 million worth of fake goods intercepted at the Port of Felixstowe in October.Border Force officers at Felixstowe stopped a shipment of fake designer luggage, bags and purses worth £4,818,092.They also uncovered a shipment of 7,688 pairs of fake UGG boots worth £920,160, and a fake load of Hermes which would have a genuine
retail value of £120,000.
Passaic County Man Arrested in Multi-Year, International Counterfeit Goods Trafficking Scheme
A Passaic County, New Jersey man was arrested today on charges that he participated in a multi-year, international conspiracy to import and sell counterfeit goods, U.S. Attorney Paul J. Fishman announced. Aref Abuhadba, 49, of Totowa, New Jersey, self-surrendered on 12th morning to special agents of the FBI on a federal criminal complaint charging him with conspiring to traffic in counterfeit goods. According to the complaint unsealed from 2003 through 2010, Abuhadba and others allegedly conspired to import counterfeit Nike sneakers and counterfeit Walt Disney-brand comforters and blankets from the People’s Republic of China (PRC) for resale in the United States. Abuhadba worked with a co-conspirator in the PRC who acted as a middleman between the manufacturers of counterfeit goods in the PRC and Abuhadba in the United States. The co-conspirator purchased counterfeit goods from manufacturers and arranged for containers of counterfeit goods to be shipped from the PRC to various ports of entry within the United States. Once the containers arrived in the United States, other co-conspirators arranged for them to be delivered to warehouses and other locations throughout the United States controlled by Abuhadba. Abuhadba then distributed the counterfeit goods to customers throughout the United States. In addition to distributing and selling counterfeit goods, Abuhadba was also responsible for collecting money from customers and wiring the proceeds of the scheme to the PRC co-conspirator and elsewhere. For his participation, Abuhadba received a fee of up to $42,000 for each container that was successfully imported into the United States. If a container was seized by law enforcement, however, Abuhadba was sometimes responsible for a portion of the costs of the goods in the seized container. According to e-mails reviewed by law enforcement during the investigation, the defendant and his co-conspirators imported at least 70 containers of counterfeit Nike sneakers and Walt Disney products. In late 2008, a number of these containers were seized by U.S. Customs and Border Protection and their contents were valued in the millions. On September 17, 2008, CBP officers at Los Angeles/Long Beach Seaport in Long Beach, California, inspected a container destined for Abuhadba in New Jersey. It was found to contain more than 10,000 pairs of counterfeit Nike Air Force One sneakers bearing various Nike trademarks. The cost of the goods seized was $201,600, and their retail value was approximately $1.5 million. Following the 2008 seizures, Abuhadba exchanged numerous e-mails with the PRC co-conspirator discussing the seizures and encouraged the PRC co-conspirator to send false letters to CBP concerning the seizures, stating that the seized containers were misdelivered and were not intended for Abuhadba. The count with which the defendant is charged carries a maximum penalty of five years in prison and a fine of up to $250,000, or twice the gross amount of gain or loss sustained by any victim.U.S. Attorney Fishman credited special agents of the FBI, under the direction of Special Agent in Charge Michael B. Ward in Newark, with the investigation leading to today’s arrest. He also thanked officers of Customs and Border Protection, under the direction of Director of New York Field Operations Robert E. Perez, for their role in the case.
Lear MoreFake goods seized in Darlington
Darlington police seized thousands of pounds worth of fake designer items in a raid at a house on the Red Hall estate. The counterfeit goods included Gucci, Chanel, Bulgari and Armani-branded watches as well as fake Louis Vuitton and Gucci handbags, counterfeit Ugg boots and Barbour jackets. A man pleaded guilty to possession of counterfeit goods with a view to selling them at Darlington Magistrates’ Court last month and was fined £640. The goods, thought to amount to several thousands of pounds in value, were handed to Darlington Council’s trading standards team for investigation and will now be destroyed. Chris McEwan, Darlington Council’s cabinet member leading on trading standards, said: “Making and selling counterfeit goods is not a victimless crime – these goods, and the people who sell them on, have a huge impact on legitimate traders who are trying to go about their business in today’s tough times. People buying the items are also possibly at risk – who knows what quality and standards the items are. “The hard work of the police and trading standards have made sure these fake goods will be off the streets and destroyed but the sad fact is more could be in circulation already.
www.thenorthernecho.co.uk/news/…/10038288.Fake_goods_seized
Lear MoreOtterBox Wins Favorable Judgment in Counterfeiting Case
OtterBox® has become one of the fastest-growing private companies in America, despite a sluggish economy. The company is recognized globally for providing premiere protective solutions for handheld technology and counterfeiters make attempts every day to replicate the Colorado-based company’s successes. OtterBox, taking necessary action to combat infringements and protect its intellectual property to the fullest extent of the law, was recently granted a $10 million judgment against a major Texas-based counterfeiting ring. The recent judgment granted by Judge Dean D. Pregerson on Oct. 19, 2012 includes a significant monetary award plus substantial attorney’s fees for the sale of at least 20,000 counterfeit OtterBox Defender Series® cases from one eBay seller over the past 10 months. The judgment also carries a permanent injunction against the seller from pursuing similar conduct in the future. All remaining inventory is to be supplied to OtterBox and all financial institutions are required to freeze activity they may have ongoing with the seller. “As the OtterBox brand continues to strengthen, we will see infringement become a prolific, constant battle,” OtterBox Founder and Chairman Curt Richardson said. “For us, protecting intellectual property has become another cost of doing business, right alongside materials, labor and shipping. We are prepared and vigilant for an ongoing, but very vital, battle.”OtterBox is working with federal and state law enforcement not only in the United States but across Europe, China and Australia to stop the detrimental manufacturing and trade of counterfeit cases.“We are one of the most popular counterfeited brands worldwide,” OtterBox Brand Protection Manager John McKinney said. “We take aggressive action to protect our brand and ensure customers are purchasing authentic, quality OtterBox product.”
Lear More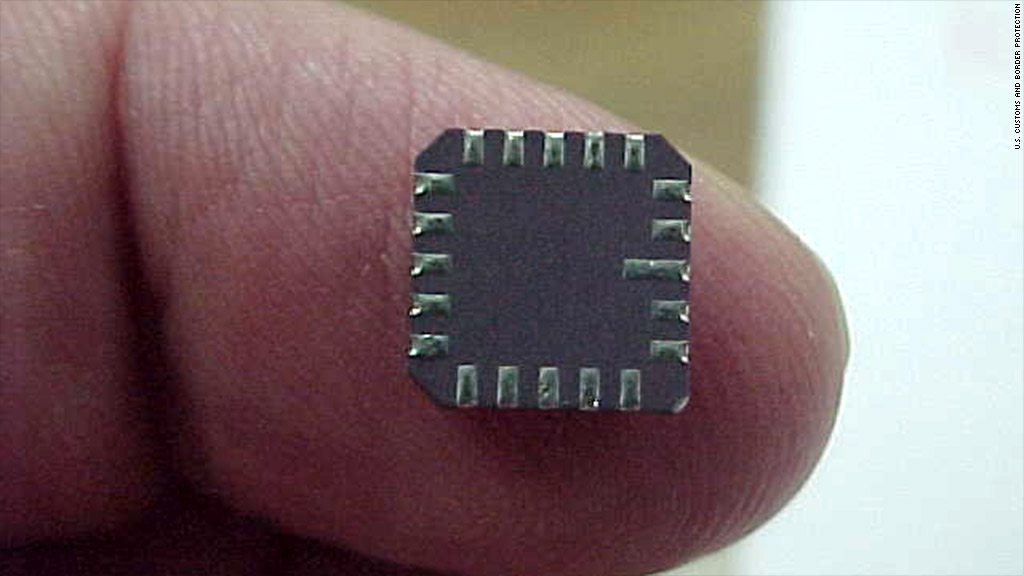
Fake tech gear has infiltrated the U.S. government
This counterfeit microchip for military use was seized by U.S. Customs and Border Protection. It’s one of a growing number of fake tech products inadvertently bought by the government. A record number of tech products used by the U.S. military and dozens of other federal agencies are fake. That opens up a myriad of national security risks, from dud missiles to short-circuiting airplane parts to cyber espionage. Despite laws designed to crack down on counterfeiters, suppliers labeled by the U.S. government as “high risk” are increasing their sales to federal agencies. Their presence in government’s supply chain soared 63% over the past decade, according to a new study released by IHS, a supply chain management consultancy. Suppliers with the high-risk branding are known to engage in counterfeiting, wire fraud, product tampering and a laundry list of other illicit and illegal behaviors. Last year, 9,539 banned businesses were found to have sold technology the government. Roughly 10% of those incidents involved counterfeit parts or equipment.”What keeps us up at night is the dynamic nature of this threat, because by the time we’ve figured out how to test for these counterfeits, they’ve figured out how to get around it,” said Vivek Kamath, formerly the head of Raytheon’s (RTN, Fortune 500) supply chain operations. “It’s literally on almost a daily basis they change. The sophistication of the counterfeiting is amazing to us.”The number of fake tech products floating around in the market quadrupled from 2009 to 2011, according to IHS — and they’re sneaking into some high-profile places. In September 2010, the Missile Defense Agency found that the memory in a high-altitude missile’s mission computer was counterfeit. Fixing the problem cost $2.7 million. Had the bomb launched, it most likely would have failed, the agency said. Two years earlier, the FBI seized $76 million of counterfeit Cisco (CSCO, Fortune 500) routers that the Bureau said could have provided Chinese hackers a backdoor into U.S. government networks. A number of government agencies bought the routers from an authorized Cisco vendor, but that legitimate vendor purchased the routers from a high-risk Chinese supplier. China continues to be the largest source for counterfeit and pirated goods found in the United States, accounting for 62% of the $178 million in products (with an estimated retail value of $1.1 billion) that the U.S. Customs and Border Protection agency seized last year. Some in Congress have pushed for a crackdown.”Counterfeit parts pose an increasing risk to our national security, to the reliability of our weapons systems and to the safety of our men and women in uniform,” Sen. John McCain, a Republican from Arizona, said last year in support of anti-counterfeiting regulations.
http://money.cnn.com/2012/11/08/technology/security/counterfeit-tech/
Lear More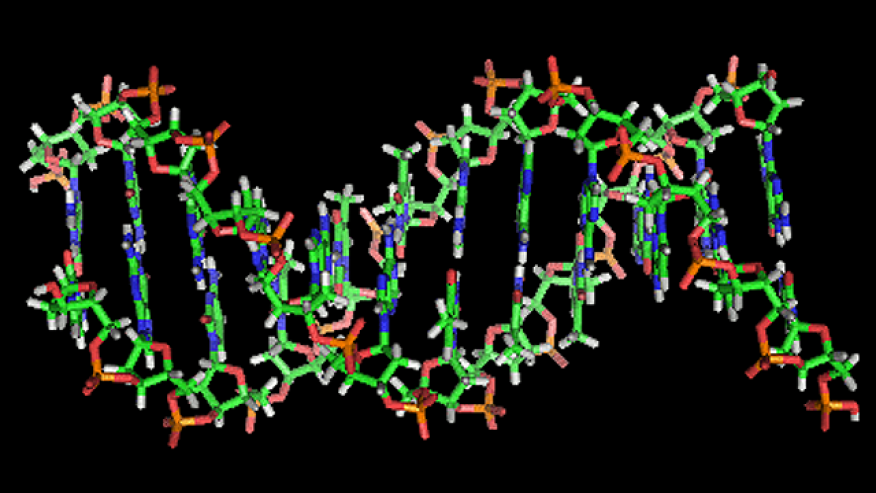
Military Uses DNA to Tackle Counterfeit Gear
Cutting corners with substandard or counterfeit electronics won’t be as easy for suppliers whose parts end up with the U.S. military, as the Department of Defense turns to DNA “barcodes” to track components. In the next month, certain kinds of electronic components sold to the military will have to be tagged with an artificial DNA sequence, which will, its designers say, make it well nigh impossible to ship a fake piece of equipment. For the military it’s a big issue, because the chips that go into a fighter jet, helicopter or infrared night vision goggles are built to exacting specifications. If the circuits don’t work, a plane might not fly — or worse, fly with a malfunctioning piece of vital equipment. In November 2011, a Senate Armed Services Committee investigation found 1,800 instances of suspect parts, and the committee grilled contractors about their supply chains. Stony Brook, N.Y.-based Applied DNA Sciences, working with the Defense Logistics Agency, offered a solution that was originally applied to textiles: plant DNA. The DNA is incorporated into the ink that gets printed on the top of the chip. Shining a laser light on it makes it fluoresce, or glow, so it’s easy to see that the chip was tagged. But that isn’t all: the DNA tags can’t be duplicated – at least not easily – so it’s a pretty good indicator that the component came from the right factory. The reason it’s so hard to copy is the nature of DNA sequencing. DNA sequences are made of four different molecules: adenine, guanine, cytosine and thymine. They can only be connected in pairs, called base pairs, that are written as GC or AT. The base pairs line up to make the familiar double helix of DNA. To sequence DNA, one has to “amplify” it – basically dissolving it in a solution and then adding chemicals to make the sequences duplicate themselves. In a living organism, DNA sequences come in certain patterns – the placement of GC and AT molecular structures are not random. When they are sequenced, a scientist can figure out what order they are supposed to be in. From there, she can say what proteins the DNA codes for. Applied DNA Sciences took the base pairs and scrambled them so that the order is essentially random. With enough base pairs, it yields millions of possible combinations. “We’ve assembled it to break all kinds of natural law,” said Karim Berrada, director of DNA formulations at Applied DNA Sciences.The number of possible combinations is quite large even for a small number of base pairs: For any bit of A, G, C, or T there are four possibilities for the molecule connected to it, so a string of, say, ten bases would have just over a million possible combinations. Berrada noted that a string of 100 base pairs have on the order of 10 to the twenty-third power arrangements.If one were to try and sequence it without knowing the proper order of base pairs, the results would be meaningless.Rory King, director at IHS iSuppli, noted that while it’s a great technology, it isn’t clear what kind of a dent it will make in the market for counterfeit consumer electronic gear, since the military makes up a relatively small part – about 1 percent — of the market. But it does help address the problem of counterfeiters using the same distribution system as legitimate manufacturers.“It gets to the issue of authentic and quality product,” King said.
http://www.foxnews.com/tech/2012/11/05/military-uses-dna-to-tackle-counterfeit-gear/
Lear MoreTanzania: State Insists TPI Made Counterfeit HIV/Aids Drugs
The government insisted that the Arusha-based Tanzania Pharmaceutical Industries (TPI) manufactured the fake anti-retroviral (ARV) drugs that were discovered in August, this year. The Deputy Minister for Health and Social Welfare, Dr Seif Rashid, told the National Assembly here that the TPI, which had its production activities suspended by the Tanzania Food and Drugs Authority (TFDA) following the allegations, sold fake ARVs branded TT-VIR 30 to the Medical Stores Department (MSD). “The documents that have been found at the MSD show that the TPI sold fake TT-VIR 30 drugs that were manufactured in batch number 0C.01.85,” he said. He said that the fake life saving drugs batch was made of tablets with three different colours, yellow, white and belayed tablets (white and pink). “Tablets with yellow colour were made up of Efaverenz instead of Nevirapine, Lamivudine and Stavudine which were supposed to be the genuine contents of the drug. The white and the combination of pink and white (belayed tablets) drugs had genuine contents (Nevirapine, Lamivudine and Stavudine) as shown in the label,” he said. The government’s statement in the House comes days after the TFDA Director General, Mr Hiiti Sillo, told journalists in Dar es Salaam that contrary to TPI’s denials, his authority has documents and exhibits that prove that the company was behind the production of the ARVs. “The documents and exhibits have been submitted to security organs for further action,” he stated. He reassured the public that the company has been suspended from manufacturing ARVs through a letter with reference number CA/C.80/222/01A/47 of October 4 this year. He also said that the authority has suspended the supply of the drugs through a letter with reference number CA/C.80/222/01A/55 of October 10, this year. Mr Sillo also denied reports that TPI is continuing with production of the drugs, noting that inspection conducted by TFDA on October 23, this year, confirmed that there was no production of the drugs. For all this time, the TPI has been insisting that it did neither manufacture nor selling of the said fake drugs. “We would like to categorically distance ourselves from the allegations. The said drugs did not emanate from TPI and are made using technology which we do not have in our factory,” said TPI Executive Director, Mrs Zarina Madabida. She said that TPI produces TT-VIR 30 in the form of oval shaped tablets whereas the fake drugs are round TT-VR 30. “We do not have the technology that can produce tablets in a round shape and in two colours. The product is simply not ours,” she said. The government has also suspended three officers pending investigations at the MSD.
http://allafrica.com/stories/201211060069.html
Lear MoreCoach Wins $257 Million Court Ruling against Counterfeiters
Coach Inc. (COH), the largest U.S. luxury handbag maker, said it won a $257 million default judgment in a lawsuit filed against businesses that operate websites selling counterfeit merchandise. The judgment also awards Coach ownership of 573 Internet domain names, the company said Nov. 2 in a statement. The docket in the case, filed in federal court in Chicago, shows that the decision was rendered Oct. 15. “This judgment should serve as a warning to everyone involved in any aspect of trafficking in counterfeit goods that Coach will find you and will seek to impose the harshest penalties available against you,” Coach’s general counsel, Todd Kahn, said in the statement. The judgment is the latest victory in Coach’s anti- counterfeiting campaign known as “Operation Turnlock,” the company said. Since the program’s inception in 2009, Coach has obtained monetary awards against manufacturers, wholesalers, flea market operators and other links in the counterfeit distribution chain, the New York-based company said. The case is Coach Inc. v. Does 1-573, 12-cv-01514, U.S. District Court, Northern District of Illinois (Chicago).
Lear More
Counterfeit incidents maintain record rate in 2012: IHS
Counterfeit parts are an escalating global supply chain challenge where a single occurrence represents widespread risk to product cost and quality, human safety, and national security. These new counterfeit report figures arrive at a time when the U.S. Department of Defense (DoD) is scheduled to update the Defense Federal Acquisition Regulation (DFAR) Supplement to the Federal Acquisition Regulation (FAR) on October 3, 2012. These updates are part of measures intended to regulate the detection and avoidance of counterfeit electronic parts as part of the National Defense Authorization Act (NDAA) of 2012. “Counterfeit parts represent a serious and growing risk to the electronics supply chain in general and to the aerospace and defense industry in particular,” said Rory King, director, supply chain product marketing at IHS. “Each month that passes, more than a hundred counterfeit incidents comprised of thousands of suspect parts are reported. That’s why the spotlight is shining squarely on tighter policies and procedures aimed at counterfeit detection and avoidance. The good news from all this attention is that an increasing number of supply chain participant companies are joining credible anti-counterfeiting organizations like ERAI – exclusive partner to IHS and an organization that monitors, investigates, and reports on counterfeit electronic components – and filing a greater number of reports that then serve as proactive alerts to others in the supply chain of real counterfeits in circulation.” Among all reporting entities in IHS figures, sources include the Government-Industry Data Exchange Program (GIDEP) and ERAI. Consistent with 2011, ERAI represents the significant majority of reports made – 88 percent of year-to-date 2012 totals. Counterfeit parts are an escalating global supply chain challenge where a single occurrence represents widespread risk to product cost and quality, human safety, and national security. These new counterfeit report figures arrive at a time when the U.S. Department of Defense (DoD) is scheduled to update the Defense Federal Acquisition Regulation (DFAR) Supplement to the Federal Acquisition Regulation (FAR) on October 3, 2012. These updates are part of measures intended to regulate the detection and avoidance of counterfeit electronic parts as part of the National Defense Authorization Act (NDAA) of 2012. “Counterfeit parts represent a serious and growing risk to the electronics supply chain in general and to the aerospace and defense industry in particular,” said Rory King, director, supply chain product marketing at IHS. “Each month that passes, more than a hundred counterfeit incidents comprised of thousands of suspect parts are reported. That’s why the spotlight is shining squarely on tighter policies and procedures aimed at counterfeit detection and avoidance. The good news from all this attention is that an increasing number of supply chain participant companies are joining credible anti-counterfeiting organizations like ERAI – exclusive partner to IHS and an organization that monitors, investigates, and reports on counterfeit electronic components – and filing a greater number of reports that then serve as proactive alerts to others in the supply chain of real counterfeits in circulation.” Among all reporting entities in IHS figures, sources include the Government-Industry Data Exchange Program (GIDEP) and ERAI. Consistent with 2011, ERAI represents the significant majority of reports made – 88 percent of year-to-date 2012 totals.
Lear More
Dealers to Join Fight Against Fake Drugs – NAFDAC
The NAFDAC has urged patent medicine dealers to join its campaign against fake and counterfeit drugs. The Director General of the agency, Dr Paul Orhii, made the call in Abakaliki on Friday in a message to a workshop designed to expose tricks of fake drug peddlers. He said the training was aimed at enlightening proprietary medicine dealers on the menace of fake drugs on public health. Orhii called for concerted efforts from all concerned in the battle against fake and regulated products. “The fight against fake and regulated products is not for NAFDAC alone. It is a fight for all so that everyone should contribute,’’ he said. Outlining the ways the stakeholders could contribute in the fight against fake and regulated products, Orhii said buying medicines from authenticated sources and obtaining letter-headed receipts of purchases could help. He said they should also ensure that they bought and sold only genuine medicines duly registered by NAFDAC. He said all adverse drug reactions should be similarly reported to NAFDAC. In his address, the Commander of the NDLEA in Ebonyi, Dr Ngozi Madubuike, described the workshop as apt in the effort to safeguard the health of Nigerians. “Fake and substandard regulated products as we all know portends very serious health hazards to those who happen to be victims. “There is no doubt that thousands of Nigerians have lost their lives due to fake and substandard regulated products. “Nigeria does not have enough pharmacists to cater for her teeming population, especially in the rural areas, hence the need to use patent medicine dealers to wage the war against fake drugs,” he said.
Lear More



