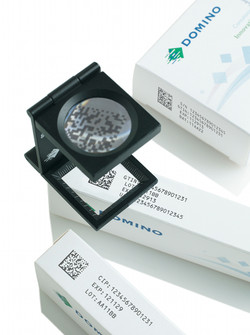
Viewpoint: A shot in the arm for generics producers
Domino inksThere is nowhere to hide from the Falsified Medicines Directive. It impacts generics producers in the same way as any other drug manufacturer or re-packager selling into Europe. With just under 48 months to go before the deadline, which is likely to be mid-2018, the clock is ticking for all producers to get their house in order.
The subject was hot on the agenda at the European Generics Association (EGA) meeting in Madrid earlier this month. As generics manufacturers often supply products with much lower margins than patented drug producers, their budgets are restricted when it comes to new investment – which is why many of them have been holding out until the last minute to find the ‘best-fit’ solution for their businesses.
Related Posts
Four busts at IGI airport: Gold smuggling, now in fruit drink mixture
For the last two months, 30-year-old Sahansha had been desperately looking for an...
Man sold fake Cisco devices worth $1 billion, ran 19 companies to list products on Amazon, eBay
A man who controlled 19 businesses, at least 15 Amazon stores, 10 eBay...
Tobacco products seized
Huge quantities of tobacco products were seized from a vehicle heading towards...
Coalition Welcomes Final Passage of Anti-Contraband Tobacco Legislation
The National Coalition Against Contraband Tobacco (NCACT) today welcomed final...




